
Three Takeaways from New FDA Report
Taxpayers Protection Alliance
December 9, 2022
The Food and Drug Administration (FDA) is facing a crisis of credibility. Taxpayers and consumers are demanding answers after contaminated infant formula left nine infants dead and millions of families without access to formula. This tragedy was just the latest in a long line of foodborne illness outbreaks which, on average, result in 3,000 deaths and 120,000 hospitalizations per year. To address the situation, the FDA tasked the non-profit Reagan-Udall Foundation to assess the agency’s shortcomings and produce a report with recommended reforms. Reagan-Udall delivered, producing a scathing report that urged a complete agency revamp. Below, the Taxpayers Protection Alliance (TPA) provides three key takeaways from the report:
No Focal Point of Responsibility
The Foundation provides readers with an organizational chart of the FDA, and it’s easy to see where the agency is falling short.
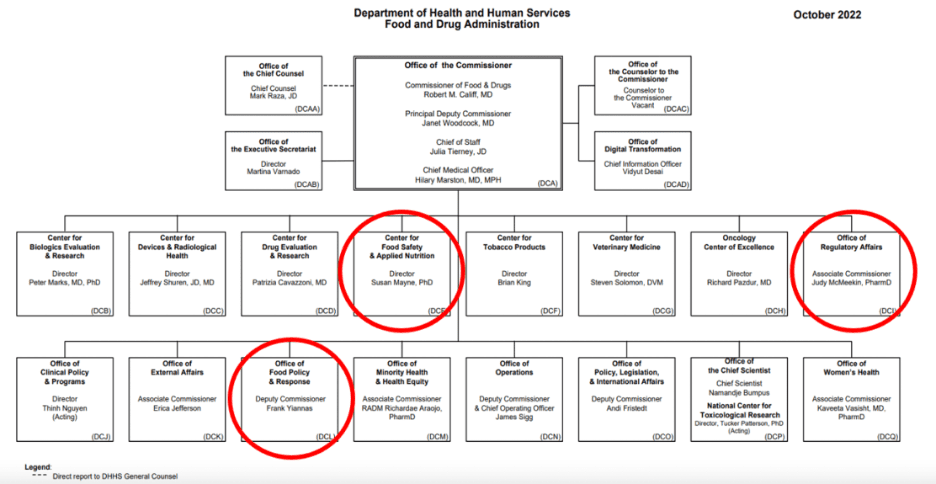
The issue is, “[t]here is no clear Human Foods Program leader or decision-maker, outside of the Commissioner. Although the missions of CFSAN [the Center for Food Safety and Applied Nutrition] and OFPR [the Office of Food Policy & Response] have differences on paper, staff are often left wondering which program is responsible for decision-making.”
The lack of overall integration can make a key difference in a food safety crisis in which the agency needs to pull many different levers at once. The infant formula crisis not only required recalls and continuous testing, but also relaxing requirements on foreign products to alleviate shortages. Coordinating all these steps under the same roof would have saved time and lives and provided peace of mind to millions of families.
Lack of User Fees
Many of the FDA’s regulatory activities are funded by user fees. Reagan-Udall notes that industry user fees account for 46 percent of the FDA’s overall budget and about two-thirds of drug evaluation and monitoring costs. Yet, strikingly, only about 1 percent of the Foods Program budget comes from user fees. Taxpayers are footing the bill for virtually all the program’s $1.1 billion budget. While user fee systems have plenty of issues, they at least ensure that companies are paying for their own products’ evaluations rather than those who may not benefit from the products. Additionally, user fees allow the FDA to quickly ramp up or redirect evaluation/inspection resources without having to wait for the appropriations process.
In its 2019 report “Reforming the FDA,” TPA recommended that the agency rely on user fees while “audit[ing] existing procedures to ensure that user fees charged to producers align with actual product evaluation costs.” TPA has also recommended procedures to keep user fees from being diverted to other programs and benefitting industries that did not pay for them. Subject to strict oversight, user fees could be a useful way for the FDA to flexibly fund its food safety mission without gouging taxpayers.
MIA: Food Innovation
The Reagan-Udall Foundation could have focused more on the role of food innovation in curbing current issues. To its credit, the Foundation does suggest an “[u]pdate to [the] application of the Delaney Clause as it relates to food additives to incorporate the application of modern toxicology approaches.” This means that food producers would no longer have to fear their product being shelved if consumer and environmental groups submit any evidence linking their product to cancer in animals. This creates an absurdly low threshold for product removal; even egg whites can be “demonstrated” to be carcinogenic in certain rodent populations.
Even if the Delaney Clause were reformed, companies would still have to face the onerous premarket food additive process. Agency approval for additives takes around six years, and sham regulatory filings by competitors may further prolong the process. This undermines food safety because new additives can considerably enhance the safety profile of foods and lower foodborne illness rates. For example, scientists can fight “food-poisoning bacteria by doctoring foods or their packaging with microbe-killing nanoparticles…” But if any of these nanoparticles require a costly FDA approval process, companies may opt for less effective safety measures such as product testing. The FDA can save lives by expediting the food additive process and capping evaluation times.
In conclusion….
The Reagan-Udall Foundation deserves plenty of credit for pointing out the FDA’s many flaws and suggesting an array of reforms. The agency should pay close attention to the report and find ways to reform its additive approval process. Hopefully, the FDA can keep food safe and put tragic foodborne illness outbreaks in the rearview mirror.
